 Français
Français Antibiotic Drugs
Cefuroxime
It is a semisynthetic, broad-spectrum second generation bactericidal cephalosporin.
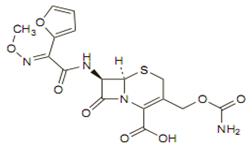
Chemical structure
Its molecular formula is C20H22N4O10S, and it has a MW of 510.48.
Mechanism of action
Mechanism of action of cefuroxime is similar to penicillin. Cefuroxime acts by inhibiting bacterial cell wall synthesis. Lack of bacterial cell wall results in death due to lysis of bacteria.
Pharmacokinetics
Cefuroxime axetil is given orally and sodium salt of cefuroxime is given by intramuscular or intravenous injection. Probenecid competes for renal tubular secretion with cefuroxime leading to higher and more prolonged plasma concentrations of cefuroxime.
Antimicrobial spectrum
Cefuroxime is bactericidal against many organisms, including many beta-lactamase–producing strains. Among second generation cephalosporin only cefuroxime crosses blood brain barrier. Cefuroxime is highly active against gram-negative cocci, gram negative bacilli, anaerobes than gram-positive cocci and gram-positive bacilli. Listeria monocytogenes and some strains of enterococci like Enterococcus faecalis and MRSA are resistant to cefuroxime.
Indications, administration and dosage
| Condition | Dosage – Adults | Dosage- children (>3 months) |
| Uncomplicated urinary-tract infections | 125 mg twice daily | 125 mg twice daily or 10 mg/kg twice daily to a maximum of 250 mg daily |
| Respiratory-tract infections | 250 to 500 mg twice daily | 30 to 60 mg/kg daily |
| Pneumonia or with acute exacerbations of chronic bronchitis |
parenteral cefuroxime 1.5 g twice daily or 750 mg twice daily, followed by 500 mg twice daily orally |
|
| Lyme disease | 500 mg twice daily for 20 days orally | 200 to 240 mg/kg daily IV in 3 or 4 divided doses, which may be decreased to 100 mg/kg daily after 3 days or after clinical improvement. |
Precautions, contraindications and warnings
Cefuroxime is contraindicated in patients with known hypersensitivity to cephalosporins and penicillins. Caution in patients receiving concomitant potent diuretics as these diuretics adversely affect renal function and patients with history of colitis. Probenecid inhibits the renal tubular secretion of cefuroxime thereby increasing its plasma levels.
Adverse reaction
Common side effects of cefuroxime include diarrhea, nausea, vomiting, transient elevation in AST, transient elevation in ALT, eosinophilia and transient elevation in LDH. Abdominal pain, cramps, headache, rash, anorexia are few rare side effects of cefuroxime.
Technical Description on Cefuroxime
It is a second generation cephalosporin.
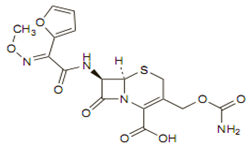
Chemical structure
Like other cephalosporins it consists of a dihydrothiazine ring fused to a beta-lactam ring containing an appropriate side chain at position 7. The molecular formula is C20H22N4O10S, and its MW is 510.48.
Preparations available
Oral (Cefuroxime axetil): 125, 250, 500 mg tablets; 125, 250 mg/5 mL suspension
Parenteral: powder to reconstitute for injection (0.75, 1.5, 7.5 g per vial or infusion pack)
Mechanism of action
Like other cephalosporins, cefuroxime possesses a mechanism of action similar to penicillins i.e. inhibition of transpeptidation process resulting in the formation of imperfect cell wall; osmotic drive from the outside isotonic environment of the host cell to the inside of the hypertonic bacterial cytoplasm and finally activation of the autolysin enzyme leading to the lysis of bacteria. Thus cefuroxime is also a bactericidal drug
Microbiology
It is active against:
Aerobic Gram-Positive
- Pneumococci
- Streptococcus pyogenes
- Staphylococcus aureus
Aerobic Gram-Negative -
- Escherichia coli
- H. influenzae
- Moraxella catarrhalis
- Haemophilus parainfluenzae
- Klebsiella pneumoniae
- Neisseria gonorrhoeae which produce beta lactamase
Spirochetel infection: Borrelia burgdorferi
Cefuroxime shows in-vitro activity against the following microorganisms:
Aerobic Gram-Positive:
- Staphylococcus epidermidis
- Staphylococcus saprophyticus
- Streptococcus agalactiae
Listeria monocytogenes, Enterococcus faecalis (strain of enterococci) and MRSA are not sensitive to cefuroxime.
Aerobic Gram-Negative:
- Proteus mirabilis
- Morganella morganii
- Proteus inconstans
- Providencia rettgeri
Anaerobic Microorganisms:
- Peptococcus niger
B. Fragilis and Clostridium difficile are not sensitive.
Resistance
Resistance to cefuroxime like other cephalosporins may be due to:
- Failure of the antibiotic to reach its site of action
- Alterations in the penicillin-binding proteins (PBPs)
This can lead to the binding of the cephalosporin to Beta –lactamases which hydrolyzes the Beta -lactam ring and inactivates the cephalosporin.
Destruction by hydrolysis of beta lactam structure is the most predominant way of resistance to cefuroxime like other cephalosporins. Enormous amounts of Beta-lactamase are released by many gram positive organisms. The gram negative bacteria produce decreased amounts of the beta lactamase enzyme but its periplasmic position makes them more capable of destructing the cephalosporins.
Cefuroxime is more resistant to hydrolysis by the beta-lactamases formed by gram-negative bacteria than first-generation cephalosporins.
Pharmacokinetics
After oral administration cefuroxime axetil is well absorbed. Esterases which are not specific present in the intestine and blood hydrolyse it to the active form cefuroxime. Cefuroxime sodium is administered by the IM or IV route. Cefuroxime has extensive tissue distribution. Inflammation of meninges allows it to pass into the CSF. It penetrates the placental barrier and is secreted during lactation.
Approximately 50% of serum cefuroxime is bound to protein. The axetil moiety is metabolized to acetaldehyde and acetic acid. Cefuroxime is eliminated without any metabolism into the urine. About50% of the oral dose is recovered in the urine within 12 hours. As cefuroxime is renally eliminated, the half-life is prolonged in patients with decreased renal function.
Therapeutic uses
1. Streptococcus pyogenes dependent pharyngitis.
Cefuroxime is normally active against nasopharyngeal streptococci.
2. Acute Maxillary Sinus infection due to pneumococci or Haemophilus influenzae which do not produce beta-lactamase.
3. Streptococcus pyogenes, Streptococcus pneumoniae caused Acute Bacterial Otitis Media and Moraxella catarrhalis and H. influenzae which produce beta lactamase.
4. Neisseria gonorrhoeae (penicillinase-producing and non-penicillinase–producing strains) dependent Uncomplicated Gonorrhoea, urethral and endocervical and uncomplicated gonorrhoea and rectal in females, caused by non-penicillinase–producing strains of Neisseria gonorrhoeae.
5. Streptococcus pneumoniae caused infections of Acute Bronchitis, Haemophilus influenzae and H. parainfluenzae (consisting of the strains which do not produce beta-lactamase).
6. Staphylococcus aureus which produce beta-lactamase or S. pyogenes caused uncomplicated skin infections.
7. Erythema Migrans due to Borrelia burgdorferi.
8. Klebsiella pneumoniae or E. coli caused Uncomplicated Urinary Tract Infections.
Dosage
The doses of cefuroxime (both sodium and axetil) are always termed as equal quantity of cefuroxime
| Condition | Dosage – Adults | Dosage- children (>3 months) |
| Uncomplicated urinary-tract infections | 125 mg twice daily | 125 mg/BD or 10 mg/kg/BD (maximal dose - 250 mg) |
| Respiratory-tract infections | 250 to 500 mg twice daily | 30 to 60 mg/kg daily |
| Pneumonia or with acute exacerbations of chronic bronchitis |
parenteral cefuroxime 1.5 g twice daily or 750 mg twice daily, followed by 500 mg twice daily orally |
|
| Lyme disease | 500 mg twice daily for 20 days orally | 200 to 240 mg/kg daily IV in 3 or 4 divided doses, which may be decreased to 100 mg/kg daily after 3 days or after clinical improvement. |
- Otitis media in children aged > two years are administered 250 mg BD or 15 mg/kg/2 times/day .The maximal dose in a day should not exceed 500 mg.
- Dosage in children (including neonates and infants) -30 to 60 mg/kg/day.
Warnings and precautions
- It can lead to super-infections.
- Clostridium difficile associated diarrhea (CDAD) can be seen with cefuroxime.
- The function of the kidneys is affected by diuretics hence cefuroxime must be cautiously used in such cases.
Special populations
- Children
On the basis of the approved use in adult population it can be safely and effectively used in children aged three months to twelve years for acute maxillary sinusitis caused by bacteria.
- Pregnancy
It is a Category B drug. Animal studies have not shown any teratogenic effects.
- Lactation
Cefuroxime should be not be used during lactation as it is secreted in breast milk.
Drug and Laboratory result interactions:
- When Fehling's or Benedict's solutions are used for estimation of urine glucose, false positive results can be seen.
Drug Interactions:
- Probenecid inhibits the renal tubular secretion of cefuroxime thereby increasing its plasma levels.
- Cefuroxime can lead to failure of oral contraceptive pills containing estrogen and progesterone due to decreased enterohepatic circulation.
Carcinogenic potential, mutagenic potential and impaired fertility:
Animal studies and in vitro studies have not shown any of these.
Contraindications
Prior history of any hypersensitive reaction to any of the cephalosporins is a contraindication to the use of cefuroxime.
Adverse reactions
The commonly observed adverse effects are:
- Diarrhea
- Vomiting
- Increased SGOT and SGPT for a limited period
- Increased levels of lactate dehydrogenase enzyme
- Increased eosinophil count
Other less commonly seen adverse effects are:
- Dyspepsia
- Pain in the abdomen along with cramps
- Decreased appetite , headache, somnolence
- Vaginal inflammation
- Skin Rashes, increased itching
- Painful micturition
- Breathlessness
- Ulcers of the oral cavity