 Français
Français Antibiotic Drugs
Cephalexin
Cephalexin is an orally active first generation bactericidal cephalosporin.
Chemical structure
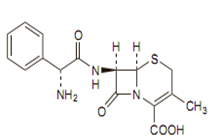
Its molecular formula is C16H17N3O4S, H2O and MW is 365.4.The chemical structure is:

Mechanism of action
Mechanism of action of cephalexin is similar to penicillin. It acts by inhibiting bacterial cell wall synthesis, lack of bacterial cell wall results in death due to lysis of bacteria.
Pharmacokinetics
Food does not interfere with absorption of cephalexin. Duration of action is prolonged in patients with impaired renal function. Probenecid delays urinary excretion of cephalexin and prolong its action.
Antimicrobial spectrum
Cephalexin is highly active against gram-positive cocci > gram negative bacilli > gram-positive bacilli > gram-negative cocci > anaerobes.
Cephalexin is not active against most strains of Enterobacter species, Morganella morganii, Proteus vulgaris, Pseudomonas species, Acinetobacter calcoaceticus and MRSA.
Indications and usage
Cephalexin is used in treatment of respiratory tract infection, otitis media, skin infections, bone infection and genitourinary infection caused by sensitive organisms. Cephalexin is normally effective in the eradication of streptococci from the nasopharynx.
Administration and dosage
| Condition | Adults | Children |
| Less severe infections | 1 to 2 g daily given in divided doses at 6-, 8-, or 12-hourly intervals | 25 to 100 mg/kg daily in divided doses |
| Severe or deep-seated infections | Up to 6 g daily | Upto 4 g daily |
For the prophylaxis of recurrent urinary-tract infection, cephalexin may be given in a dose of 125 mg at night.
Precautions, contraindication and warning
Cephalexin dose reduction is needed in patients with renal impairment. Cephalexin is contraindicated in patients with known allergy to the cephalosporin group of antibiotics. Cephalexin is avoided in porphyria. Prolonged administration of cephalexin may lead to overgrowth of nonsusceptible microorganisms.
Adverse effects
Side effects like diarrhoea, dyspepsia, abdominal discomfort, gastritis, rash, urticarial, angioedema, dizziness, fatigue, headache, agitation, confusion may occur with cephalexin.Technical Description on Cephalexin
It is an orally active first generation cephalosporin.
Chemical structure
Like other cephalosporins cephalexin consists of a dihydrothiazine ring fused to a beta-lactam ringand has a D-phenylglycyl group as a substitute at the 7-amino position and an unsubstituted methyl group at the 3-position.Its molecular formula is C16H17N3O4S, H2O and MW is 365.4.The chemical structure is:
Preparations available
Oral: 250, 500 mg capsules and tablets; 1 g tablets; 125, 250 mg/5 mL suspension
Mechanism of action
Like other cephalosporins, cephalexin possesses a mechanism of action similar to penicillins i.e. inhibition of transpeptidation process resulting in the formation of imperfect cell wall; osmotic drive from the outside isotonic environment of the host cell to the inside of the hypertonic bacterial cytoplasm and finally activation of the autolysin enzyme leading to the lysis of bacteria. Thus cephalexin is also a bactericidal drug.
Microbiology
Cephalexin is active against the following organisms:
Gram positive aerobes
- Staphylococcus aureus (including penicillinase-producing strains)
- Streptococcus pneumonia (penicillin susceptible strains)
- Streptococcus pyogenes
Gram negative aerobes
- Escherichia coli
- Hemophilus influenzae
- Klebsiella pneumonia
- Moraxella catarrhalis
- Proteus mirabilis
MRSA and most strains of enterococci like Enterococcus faecalis are resistant to cephalexin.
Also cephalexin is not active against most strains of Enterobacter species, Morganella morganii, Proteus vulgaris, Pseudomonas species and Acinetobacter calcoaceticus.
Resistance
Resistance to cephalexin like other cephalosporins may be due to:
- Failure of the antibiotic to reach its site of action
- Alterations in the penicillin-binding proteins (PBPs)
This can lead to the binding of the cephalosporin to Beta –lactamases which hydrolyses the Beta-lactam ring and inactivates the cephalosporin.
The most predominant mechanism of resistance to cephalexin like other cephalosporins is destruction of the cephalosporins by hydrolysis of the Beta-lactam ring. Enormous amounts of Beta-lactamase are released into the surrounding medium by many gram positive organisms. Though the secretion of Beta-lactamase by gram-negative bacteria is less, the position of their enzyme in the periplasmic space make it more effective in destroying cephalosporins because they diffuse to their targets on the inner membrane, as is the case for the penicillins.
Pharmacokinetics
Cephalexin is rapidly and completely absorbed orally. It can be administered without food or with food. It achieves peak plasma concentration 1 hour after oral administration.The plasma protein binding is about 15%.The plasma half-life is about 1 hour; it increases with reduced renal function. Cephalexin is extensively distributed in the body but does not enter the CSF in substantial quantities. It crosses the placenta and small quantities are found in breast milk. Cephalexin is not metabolised. The major route of elimination is renal. Cephalexin is eliminated unchanged in urine by glomerular filtration and tubular secretion. Approximately 80-90 % of the dose is excreted unchanged in the urine in 8 hours.Probenecid delays urinary excretion. Therapeutically effective concentrations may be found in the bile and some may be excreted by this route. Cephalexin is removed by haemodialysis and peritoneal dialysis.
Therapeutic uses
- Respiratory tract infections caused by Streptococcus pneumonia and Streptococcus pyogenes.
Cephalexin is normally effective in the eradication of streptococci from the nasopharynx. No data is available to validate the effectiveness of cefuroxime in the treatment of penicillin-resistant strains of Streptococcus pyogenes.
- Otitis media due to Streptococcus pneumonia, Streptococcus pyogenes, Staphylococcus aureus, H. Influenzae and Moraxella catarrhalis.
- Skin and skin structure infections caused by Staphylococcus aureus and/or Streptococcus pyogenes.
- Bone infections caused by Staphylococcus aureus and/or Proteus mirabilis.
- Genitourinary tract infections, including acute prostatitis, caused by E. coli,Proteus mirabilis and Klebsiella pneumonia.
Dosage
Cephalexin is typically given as the monohydrate although the hydrochloride is sometimes used. Doses are expressed in terms of the equivalent amount of anhydrous Cephalexin; 1.05 g of Cephalexin monohydrate and 1.16 g of Cephalexin hydrochloride are each equivalent to about 1 g of anhydrous Cephalexin.
| Condition | Adults | Children |
| Less severe infections | 1 to 2 g daily given in divided doses at 6-, 8-, or 12-hourly intervals | 25 to 100 mg/kg daily in divided doses |
| Severe or deep-seated infections | Up to 6 g daily | Upto 4 g daily |
For the prophylaxis of recurrent urinary-tract infection, cephalexin may be given in a dose of 125 mg at night.
Drug interactions
- The renal excretion of cephalexin, like other cephalosporins, is delayed by probenecid.
- Cephalexin may increase the plasma levels of metformin and decrease its renal excretion.
Special populations
- Patients with renal impairment
Doses of cephalexin may need to be reduced in patients with renal impairment. The following maximum daily doses are recommended according to creatinine clearance (CC):
• CC 40 to 50 mL/minute: maximum 3 g daily
• CC 10 to 40 mL/minute: maximum 1.5 g daily
• CC less than 10 mL/minute: maximum 750 mg daily
- Pregnancy
It is a pregnancy category B drug. Animal studies have not shown any harmful effects to foetus. But well controlled clinical trials have not been conducted in humans.
- Lactation
Cephalexin is excreted in human milk; care should be taken while prescribing it to nursing mothers.
- Paediatric use
The safety and efficacy of cephalexin has been established in children.
- Geriatric use
No general differences in safety or effectiveness were seen between elderly subjects and younger adult subjects.
Warnings and precautions
- Before initiating treatment with cephalexin, vigilant investigation should be done to determine whether the patient has had previous hypersensitivity reactions to cefuroxime, other cephalosporins, penicillins, or other drugs.
- As with other broad-spectrum antibiotics, prolonged administration of cephalexin axetil may lead to result overgrowth of nonsusceptible microorganisms.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cephalexin, and may range in severity from mild diarrhea to fatal colitis.
- Cephalexin is unsafe in porphyria
Contraindications
- Cephalexin is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
Adverse reactions
The commonly seen adverse effects are:
- Diarrhoea,dyspepsia, gastritis and abdominal pain
- Allergic reactions – rash, urticaria, angioedema, erythema multiforme and Steven Johnson syndrome
- Genital and anal pruritis, genital moniliasis, vaginitis and vaginal discharge
- Dizziness, fatigue, headache, agitation, confusion, hallucinations
- Arthralgia, arthritis, joint disorder
- Reversible interstitial nephritis
- Eosinophilia, neutropenia, thrombocytopenia, haemolytic anemia, slight elevations in AST and ALT.