 Français
Français Antibiotic Drugs
Clindamycin
Clindamycin is a semi-synthetic and belongs to lincosamide.
Chemical structure
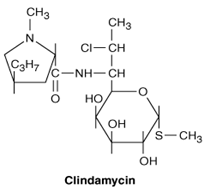
It has a molecular weight of 504.96, and the molecular formula is C18H34ClN208PS Its chemical structure is:

Mechanism of action
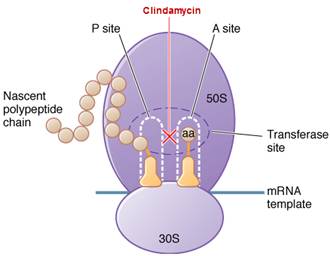
Clindamycin is bacteriostatic drug acts by inhibiting protein synthesis. It binds on the 50S subunit of the bacterial ribosome. It suppresses protein synthesis by interfering with the development of initiation complexes and with aminoacyl translocation reactions.
Site of action

Pharmacokinetics
Food does not interfere with absorption of clindamycin. Only ~10% clindamycin is excreted unchanged in the urine, and small quantities are found in the faeces. Though, antimicrobial activity continues in faeces for 5 days after parenteral therapy with clindamycin is stopped; growth of clindamycin-sensitive microorganisms in colonic substances may be inhibited for up to 2 weeks.
Antimicrobial spectrum, indications, administration and dosage
Clindamycin is active against bacteroides fragilis, P. jiroveci, T. gondii, clostridium, corynebacterium acnes, pneumocystis carinii. So it is useful for treatment of abdominal and pelvic abscess, respiratory tract infections including lung abcess, skin and soft-tissue Infections.
Oral
| Condition | Dosage | |
| Adults | Children (clindamycin palmitate HCl) | |
| Non-Severe infections | 150-300 mg 6 hourly | 8-12 mg/kg/day (3-4 divided doses) |
| Severe infections | 300-600 mg 6 hourly | 13-25 mg/kg/day |
However, children weighing 10 kg should receive 1/2 teaspoonful of clindamycin palmitate hydrochloride (37.5 mg) every 8 hours as a minimal dose.
Parenteral (Clindamycin phosphate)
| Condition | Dosage | |
| Adults | Children | |
| Severe infections | 1200-2400 mg/day (3-4 divided doses) | 15-40 mg/kg/day (3-4 divided doses) |
Daily doses as high as 4.8 g have been given intravenously to adults. In children, a minimal daily dose of 300 mg is recommended, regardless of body weight.
Precautions, contraindications and warning
It is contraindicated in patients with known hypersensitivity to clindamycin or lincomycin.
Adverse effects
Diarrhoea may occur in 2-20% of patients receiving clindamycin. Other side effects are nausea, vomiting, abdominal cramps, metallic taste, neuromuscular blockade, rashes, stomatitis and urticarial.
Technical Description on Clindamycin
Clindamycin is a chlorine-substituted derivative of lincomycin, an antibiotic that is elaborated by Streptomyces lincolnensis. It issemi-synthetic and belongs to lincosamide class. It isa derivative of the amino acid trans-L-4-n-propylhygrinic acid, attached to a sulfur-containing derivative of an octose. It has a molecular weight of 504.96, and the molecular formula is C18H34ClN208PS Its chemical structure is:
Preparations available
Oral: 75, 150, 300 mg capsules; 75 mg/5 mL granules to reconstitute for solution.
Parenteral: 150 mg/mL in 2, 4, 6, 60 mL vials for injection.
Clindamycin is also available as a topical solution, gel, or lotion and as a vaginal cream.
Mechanism of action
The binding site for clindamycin is on the 50S subunit of the bacterial ribosome which is identical with that for erythromycin. Clindamycin, like erythromycin, suppresses protein synthesis by interfering with the development of initiation complexes and with aminoacyl translocation reactions. Although clindamycin, erythromycin, and chloramphenicol are not structurally linked, they act at sites in close vicinity, and binding by one of these antibiotics to the ribosome may inhibit the interaction of the others.
Site of action
Antimicrobial Activity
- Clindamycin is usually similar to erythromycin in its in vitro activity against susceptible strains of pneumococci, S. pyogenes, and viridans streptococci.
- Methicillin-susceptible strains of S. aureus generally are susceptible to clindamycin, but methicillin-resistant strains of S. aureus and coagulase-negative staphylococci frequently are resistant.
- Clindamycin is more active than erythromycin or clarithromycin against anaerobic bacteria, especially B. fragilis.
- Other bacteroides species and other anaerobes, both gram-positive and gram-negative, like Bacteroides melaninogenicus, Peptostreptococcus, Peptococcus and C. perfringens are usually susceptible.
- Resistance to clindamycin in Bacteroides species is rapidly increasing. Strains of Actinomyces israelii and Nocardia asteroides are sensitive. Chlamydia species are variably sensitive.
- Essentially all enterococci, aerobic gram-negative organismsand M. pneumoniaeare resistant.
- Clindamycin has excellent activity against Corynebacterium acnes.
- Clindamycin plus primaquine and clindamycin plus pyrimethamine are second-line regimens for Pneumocystis jiroveci pneumonia and T. gondii encephalitis, respectively.
- Clindamycin is useful in the treatment of babesiosis.
Resistance
Resistance to clindamycin, which usually confers cross-resistance to macrolides, is due to (1) mutation of the ribosomal receptor site; (2) modification of the receptor by a constitutively expressed adenine methylase ; and (3) enzymatic inactivation (plasmid mediated adenyl transferase) of clindamycin. Gram-negative aerobic species are innately resistant because of poor permeability of the outer membrane. Resistance due to ribosomal methylation by erm-encoded enzymes in macrolides may also produce resistance to clindamycin. There is cross-resistance only if the enzyme is formed constitutively, as clindamycin does not induce the methylase. Conversely, strains with inducible resistance may develop constitutive production of the methylase during therapy. Hence use of clindamycin should be avoided in the treatment of deep-rooted infections caused by organisms exhibiting an inducible resistance phenotype. Detection of this phenotype can be done by approximating erythromycin and clindamycin on an agar plate with a lawn of the organism; a blunting of the zone of inhibition between clindamycin and erythromycin suggests inducible resistance (this is known as the "D-test"). Clindamycin is not a substrate for macrolide efflux pumps; so strains that are resistant to macrolides by this mechanism are susceptible to clindamycin. Infrequently altered metabolism causes clindamycin resistance. Resistance commonly occurs via macrolide -lincosamide-streptogramin B (MLSB) type of resistance which may be constitutive or inducible.
Pharmacokinetics
Clindamycin is nearly completely absorbed orally. Peak plasma concentrations are attained within 1 hour after 150 mg dose. Food does not reduce absorption significantly. The half-life is about 2.5-2.9 hours in normal individuals, increasing to 6 hours in patients with anuria. Clindamycin palmitate, an oral formulation for pediatric use, is an inactive prodrug that is hydrolyzed rapidly in vivo. Its rate and magnitude of absorption are comparable to those of clindamycin.The phosphate ester of clindamycin, which is given parenterally, is also promptly hydrolyzed in vivo to the active parent compound. After intramuscular injection, peak concentrations are not achieved until 3 hours in adults and 1 hour in children.
Clindamycin penetrates well into most tissues including bone, with brain and cerebrospinal fluid being important exceptions. Itfreely crosses the placenta. Plasma protein binding is > 90 %. It penetrates well into abscesses and is actively taken up and concentrated by phagocytes, polymorphonuclear leukocytes, and alveolar macrophages.
Only ~10% clindamycin is excreted unchanged in the urine, and small quantities are found in the faeces. Though, antimicrobial activity continues in faeces for 5 days after parenteral therapy with clindamycin is stopped; growth of clindamycin-sensitive microorganisms in colonic substances may be inhibited for up to 2 weeks.
Clindamycin is inactivated by metabolism to N-demethylclindamycin and clindamycin sulfoxide, which are excreted in the urine and bile. Build-up of clindamycin can occur in severe hepatic failure, and dosage adjustments may be essential. No dosage adjustment is required for renal failure.
Therapeutic Uses and Dosage
Oral
| Condition | Dosage | |
| Adults | Children(clindamycin palmitate HCl) | |
| Non-Severe infections | 150-300 mg 6 hourly | 8-12 mg/kg/day (3-4 divided doses) |
| Severe infections | 300-600 mg 6 hourly | 13-25 mg/kg/day |
However, children weighing 10 kg should receive 1/2 teaspoonful of clindamycin palmitate hydrochloride (37.5 mg) every 8 hours as a minimal dose.
Parenteral (Clindamycin phosphate)
| Condition | Dosage | |
| Adults | Children | |
| Severe infections | 1200-2400 mg/day (3-4 divided doses) | 15-40 mg/kg/day (3-4 divided doses) |
Daily doses as high as 4.8 g have been given intravenously to adults. In children, a minimal daily dose of 300 mg is recommended, regardless of body weight.
Therapeutic uses
1. Use of clindamycin is mainly for anaerobic and mixed infections, especially by Bacteroides fragilis causing abdominal and pelvic abscess. It is generally combined with aminoglycoside or cephalosporin.
2. Respiratory Tract Infections
- Clindamycin (600 mg IV 8 hourly) has replaced penicillin as the drug of choice for treatment of lung abscess and anaerobic lung and pleural space infections.
- Clindamycin (600 mg IV 8 hourly, or 300-450 mg orally 6 hourly for less severe disease) in combination with primaquine (15 mg of base once daily) is beneficial for the treatment of mild to moderate cases of P. jiroveci pneumonia in AIDS patients.
3. Skin and Soft-Tissue Infections
Clindamycin is used as an alternative, especially in patients with -lactam allergies as it has decent activity against aerobic and anaerobic gram-positive cocci and high oral bioavailability. It has also been used for the treatment of necrotizing skin and soft-tissue infections as it diminishes toxin expression.
4. Other Infections
- Acute treatment of encephalitis caused by T. gondii in patients with AIDS - Clindamycin (600-1200 mg IV 6 hourly) + pyrimethamine (200-mg loading dose followed by 75 mg orally each day) and leucovorin (folinic acid, 10 mg/day) is effective.
- It is effective topically (or orally) for acnevulgaris and bacterial vaginosis.
- Bone and joint infections due to anaerobic streptococci and Clostridium perfringens.
- It can also be used for prophylaxis of endocarditis in penicillin allergic patients with valvular defects who undergo dental surgery.
- It can be used to prevent surgical site infection in colorectal/pelvic surgery.
Drug interactions
- Clindamycin can inhibit neuromuscular transmission and may potentiate the effect of a neuromuscular blocking agent administered concomitantly.
- Agents that inhibit peristalsis, such as opioids, may extend and deteriorate pseudomembranous colitis associated with clindamycin.
- Clindamycin, erythromycin and chloramphenicol display mutual antagonism, probably because their ribosomal binding sites are in close proximity; binding of one hinders access of the other to its target site.
Contraindication
It is contraindicated in patients previously found hypersensitive to clindamycin or lincomycin.
Pregnancy and fertility
Animal studies have not proved any teratogenic effects or adverse effects on fertility.
Lactation
It is excreted in breast milk, caution is advised in lactation.
Adverse Effects
1. Gastrointestinal Effects
Incidence of diarrhea with clindamycin is 2% to 20%. Variable numbers of patients (0.01-10%) have developed pseudomembranous colitis which is characterized by watery diarrhea, fever, and elevated peripheral WBC counts.
Cessation of the drug, combined with administration of metronidazole or oral vancomycin usually is curative, but relapses occur in up to 20% of cases.
2. Other Effects
- Nausea, vomiting, abdominal cramps, metallic taste.
- High IV doses can lead to neuromuscular blockade like aminoglycosides.
- Hypersensitivity reactions like rashes, stomatitis and urticaria can occur.
- Skin rashes occur in ~10% of patients treated with clindamycin and may be more common in patients with HIV infection.
- Rare effects include exudative erythema multiforme (Stevens-Johnson syndrome), reversible elevation of AST and ALT, granulocytopenia, thrombocytopenia, and anaphylactic reactions.
- Local thrombophlebitis may follow intravenous administration of the drug.