 Français
Français Antibiotic Drugs
Fidaxomicin
Fidaxomicin is a macrocycle antibiotic and is classified as a macrolide. It is derived from the fermentation products of Actinoplanes deccanensis and Dactylosporangium aurantiacum subspecies hamdenensis.
Chemical structure
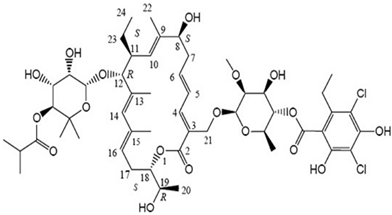
Its molecular formula is C52H74Cl2O18 and MW is 1058.04.
Mechanism of action
Fidaxomicin is thought to inhibit bacterial protein synthesis through non-competitive inhibition of transcription by binding to bacterial RNA polymerase. Fidaxomicin binds to σ subunits and due to this distinctive target site it has limited scale of antimicrobial activity and so has not reported cross-resistance with other antibiotic classes.
Pharmacokinetics
Fidaxomicin when given orally has negligible absorption and mainly remains confined to and acts locally in the GI tract. Fatty meal may increase absorption.
Antimicrobial spectrum
Fidaxomicin has a narrow range of antibacterial activity due to its action on distinctive target site. It is active against certain gram-positive bacteria. It has restricted or no activity against gram-negative bacteria and Candida albicans. It is bactericidal when used for the treatment of C. difficile, with a low tendency for resistance development, no cross-resistance to current antibiotics, and an extended post-antibiotic effect.
Indications and uses
Fidaxomicin is used for treatment of Clostridium difficile-associated diarrhea (CDAD) in adult’s ≥18 years of age. It has been nominated as an orphan drug by FDA for treatment of C. difficile infection (CDI) in paediatric patients.
Administration and dosage
Adults
| Condition | Dose (oral) |
| Clostridium difficile-associated Diarrhea | 200 mg twice daily for 10 days. |
Precautions, contraindications and warnings
Fidaxomicin is not effective for treatment of systemic infections as only marginal systemic absorption occurs after oral administration.
Precaution is advised in following patients as clinical studies with fidaxomicin have not been conducted in them:
- Patients with life-threatening or fulminant CDI (WBC count > 30×103/mm3, body temperature > 40°, systolic blood pressure < 90 mm Hg, septic shock, peritoneal signs, or significant dehydration)
- Toxic megacolon
- History of ulcerative colitis or Crohn's disease
- Pregnancy (category B) or breastfeeding
- Concomitant use of other antibiotics to treat CDI
- Concomitant use of other drugs to control diarrhea or other drugs that could affect peristalsis
- More than one prior occurrence of CDI within the past 3 months
Adverse effects
Common adverse effects of fidaxomicin are nausea, vomiting, abdominal pain, gastrointestinal haemorrhage, anemia, neutropenia, hypokalemia, fever, edema. Rare side effects are abdominal distension, dyspepsia, dysphagia, flatulence, drug eruption, pruritus, rash, increased hepatic enzymes, decreased platelet count.
Technical Description on Fidaxomicin
Fidaxomicin is a macrocyclic antibiotic and is classified as a macrolide. It is derived from the fermentation products of Dactylosporangium aurantiacum subspecies hamdenensis and Actinoplanes deccanensis.
Chemical structure
Unlike other macrolides which have 14- or 15-member lactone ring structures, fidaxomicin is an 18-membered macrocyclic ester molecule with a 7-carbon sugar at position C12 and a 6-deoxysuga at C21. Its molecular formula is C52H74Cl2O18 and MW is 1058.04.

Preparations available
Tablet – 200 mg
Mechanism of action
Fidaxomicin causes inhibition of synthesis of protein. It causes this by non-competitively inhibiting the process of transcription of proteins by attachment to the bacterial enzyme RNA polymerase. The bacterial RNA polymerase is large molecule possessing 5 subunits that constitute its main part which carries out catalysis. The 5 subunits consist of two alpha units, two beta units with slight variation from each other and one ω subunit. To recognise the promoter one more isolated sigma subunit (σ) is also present. The sigma subunit is specifically inhibited by fidaxomicin. Due to this distinctive site of action fidaxomicin does not has much of an antibacterial action as various microorganisms have differing sigma subunits. This is also the reason for no cross resistance with any other class of antibacterial agents.
Microbiology
Fidaxomicin has a narrow range of antibacterial activity. It is active against certain gram-positive bacteria like Staphylococci, clostridium difficile and perfringes, and enterococci. It has restricted or no activity against gram-negative bacteria and Candida albicans. Fidaxomicin has cidal action on Clostridium difficile, with a low tendency for resistance development, no cross-resistance to current antibiotics, and an extended post-antibiotic effect.
Fidaxomicin has an active metabolite, OPT-1118, which has an antimicrobial spectrum identical to that of the parent compound, with 8–16-fold lesser activity. It has a long post-antibiotic effect (4–6 hour compared with <1 hour for vancomycin).
Resistance
In vitro studies with Clostridium difficile strains have not reported cross-resistance with Metronidazole, rifampin, the macrolide azithromycin, vancomycin, penicillins like ampicillin, and fluoroquinolone like ciprofloxacin. Fidaxomicin has synergistic effects when prescribed simultaneously with rifampin. Spontaneous mutational resistance in microorganisms is minimal with fidaxomicin. The β' subunit of the bacterial RNA polymerase enzyme undergoes point mutation sometimes which is responsible for decreased sensitivity to fidaxomicin. Not much clinical data is available about resistance in general population.
Pharmacokinetics
Only negligible systemic absorption of fidaxomicin is seen orally; it mainly remains confined to and acts locally in the GI tract. Fatty meal may increase absorption. After oral intake, fidaxomicin is metabolized to OP-1118 which the active metabolite. Due to no systemic absorption, it has been postulated that fidaxomicin is converted to OP-1118 by hydrolysis in the acidic pH of the stomach. Also microsomes present in the intestine are known to carry out this conversion.
Maximal plasma levels are achieved within one to two hours for both fidaxomicin and OP-1118. Metabolism of fidaxomicin and formation of OP-1118 is not dependent on CYP isoenzymes. They are excreted mainly in faeces. In healthy adults, greater than 92% of an oral dose is recovered in faeces as fidaxomicin and OP-1118; and less than one per cent of dose recovered in urine as OP-1118.
Fidaxomicin elimination half-life is about 12 hours and that of OP-1118 is approximately 11 hours.
Therapeutic uses
- Clostridium difficile-associated Diarrhea
It is indicated for the therapy of Clostridium difficile-associated diarrhea (CDAD) in patients greater 18 years of age.
Dosage
Adults
| Condition | Dose (oral) |
| Clostridium difficile-associated Diarrhea | 200 mg BD for ten days. |
Special Populations
- Kidney dysfunction
No modifications in the dose are required in patients with kidney dysfunction.
- Liver dysfunction
There is not much data available but fidaxomicin does not undergo metabolism in the liver to a substantial level.
- Pregnancy
It is a pregnancy category B drug.
- Lactation
It is not known whether fidaxomicin is secreted during lactation.
- Children
The efficacy and safety of fidaxomicin has not been studied in patients <18 years of age. It is designated as an orphan drug by FDA for treatment of clostridium difficile associated enterocolitis in paediatric patients.
Warnings and Precautions
- Systemic Infections
It is not effective for treatment of systemic infections as only marginal systemic absorption occurs after oral administration.
- Sensitivity Reactions
Rarely drug eruption reactions, pruritus, and skin rashes have been reported.
Precaution is advised in following patients as clinical studies with fidaxomicin have not been conducted in them:
- Patients with life-threatening or fulminant CDI
- History of inflammatory bowel disease (Crohn's disease or ulcerative colitis)
- Toxic megacolon
- Simultaneous prescription of other antibiotics to treat CDI
- Simultaneous prescription of other drugs to control diarrhea or other drugs that could affect peristalsis
- More than one prior occurrence of CDI within the past three months
Drug interactions
- The metabolism of fidaxomicin and production of the metabolite (OP-1118) is not dependent on CYP isoenzymes. Hence there is no clinically significant effect on pharmacokinetics of CYP3A4, CYP2C19, or CYP2C9, substrates and dosage alterations are not needed if fidaxomicin is prescribed simultaneously with CYP isoenzyme substrates.
- Fidaxomicin and OP-1118 are substrates of P-glycoprotein (P-gp) transport system.
But fidaxomicin can be simultaneously prescribed P-gp inhibitors without any alterations in the dose as no interactions have been found in clinical trials.
- Clinical studies suggest that fidaxomicin may be a more effective therapy for CDI in patients requiring concomitant antibiotics.
Carcinogenic, Mutagenic potential, and impaired fertility
Long-term carcinogenicity studies in animals have not been conducted to evaluate the carcinogenic potential of fidaxomicin. However, neither mutagenic nor carcinogenic potential nor impairment of fertility was found in a number of tests conducted in selected animals.
Adverse Effects
Common adverse effects observed were:
- Pain in the abdomen, vomiting
- Bleeding in the GIT
- Anemia
- Decreased neutrophil count
- Decreased serum potassium levels
- Pyrexia
Other rare adverse effects observed were:
- Distension of the abdomen, difficulty in swallowing, dyspepsia, obstruction of the intestine, flatulence
- Skin rashes, increased itching, drug eruption reactions
- Increased blood glucose levels, acidosis
- Increased blood ALP, decreased bicarbonate levels in the blood, thrombocytopenia
Continue on to the next page, Levofloxacin