 Français
Français Antibiotic Drugs
Levofloxacin (Tavanic)
Levofloxacin is a synthetic, broad spectrum, second generation bactericidal fluoroquinolone.
Chemical structure
The empirical formula is C18H20FN3O4 and MW is 361.4.

Mechanism of action
Levofloxacin inhibits the enzyme bacterial DNA gyrase and prevents replication of bacterial DNA during bacterial growth and reproduction.
Site of action

Pharmacokinetics
Levofloxacin is rapidly and almost completely absorbed orally with peak plasma concentrations occurring within 1 to 2 hours. The absolute bioavailability of levofloxacin is approximately 99-100%, demonstrating complete oral absorption of levofloxacin. Levofloxacin is stereo-chemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. The elimination half-life of levofloxacin is 6 to 8 hours, which may be prolonged in patients with renal impairment.
Antimicrobial spectrum
Levofloxacin is active against aerobic gram-positive bacteria, aerobic gram- negative bacteria, anaerobic bacteria, chlamydophila pneumonia, chlamydophila psittaci, chlamydia trachomatis, legionella pneumophila, mycoplasma pneumonia, mycoplasma hominis and ureaplasma urealyticum
Indications, administration and dosage
| Condition | Dosage |
| Acute bacterial sinusitis | 500 mg once daily for 10-14 days |
| Acute bacterial exacerbations of chronic bronchitis | 500 mg once daily for 7-10 days |
| Community-acquired pneumonia | 500 mg once or twice daily for 7-14 days |
| Pyelonephritis | 500 mg once daily for 7-10 days |
| Complicated urinary tract infections | 500 mg once daily for 7-14 days |
| Uncomplicated cystitis | 250mg once daily for 3 days |
| Chronic bacterial prostatitis. | 500 mg once daily for 28 days |
| Complicated skin and soft tissue infections | 500 mg once or twice daily for 7-14 days |
| Inhalation Anthrax | 500 mg once daily for 8 weeks |
Precautions, contraindications and warnings
Levofloxacin is contraindicated in in patients hypersensitive to levofloxacin or other quinolones or any of the excipients, in patients with epilepsy, in patients with history of tendon disorders related to fluoroquinolone administrationin children or growing adolescents, during pregnancy and in breast-feeding women.
Adverse effects
The common adverse effects seen with levofloxacin are moniliasis, insomnia, headache, dizziness, dyspnea , nausea, diarrhea, constipation, abdominal pain, vomiting, dyspepsia, rash, pruritus.
Technical Description on Levofloxacin (Tavanic)
Levofloxacin is a synthetically derived second generation fluoroquinolone.
Chemical structure
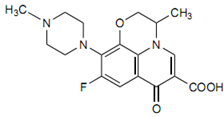
In this formulation levofloxacin is present as levofloxacin hemihydrate. It is a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The molecular formula is C18H20FN3O4 and MW is 361.4.
Preparations available
Oral – Tablets of 250/500 mg
For IV use - 5 mg/ml
Mechanism of action
Levofloxacin like other fluoroquinolones (FQs) inhibits the enzyme bacterial DNA gyrase that causes small cuts in the DNA, causes negative supercoiling and then re-ligates the cut ends. This helps in averting positive supercoiling which may occur in excess. The DNA gyrase comprises of two A and two B subunits: The A subunit leads to small cuts in the DNA, B subunit then brings about negative supercoiling and afterwards the A subunit causes re-ligation. Levofloxacin attaches to A subunit with great affinity and restricts the above mentioned function of the DNA gyrase enzyme. The predominant target in gram-positive bacteria is an analogous enzyme topoisomerase IV that nicks and separates daughter DNA strands once the DNA replication is complete. The damaged DNA leads to formation of exonucleases resulting in digestion of the DNA and this possibly contributes to the bactericidal action of levofloxacin.
The cells of mammals have the enzyme topoisomerase II instead of DNA gyrase or topoisomerase IV which possesses very little affinity for levofloxacin resulting in minimal damage to the host tissue.

Microbiology
Levofloxacin is active against the following organisms:
Aerobic Gram-positive bacteria
- Bacillus anthracis
- Staphylococcus aureus which are sensitive to methicillin.
- Staphylococcus saprophyticus
- Streptococci, group C and G and agalactiae
- Pneumococci
- Streptococcus pyogenes
Aerobic Gram- negative bacteria
- Eikenella corrodens
- Haemophilus influenzae and parainfluenzae
- Klebsiella oxytoca
- Moraxella catarrhalis
- Pasteurella multocida
- Proteus vulgaris
- Providencia rettgeri
Anaerobic bacteria
- Peptostreptococcus
Other
- Chlamydophila pneumoniae and psittaci
- Chlamydia trachomatis
- Legionella pneumophila
- Mycoplasma pneumonia and hominis
- Ureaplasma urealyticum
Resistance
Resistance is mainly because of chromosomal mutation (Quinolone-Resistance Determining Regions {QRDRs}) forming a DNA gyrase or topoisomerase IV with reduced affinity for levofloxacin. Another common mechanism is reduced permeability/increased efflux of levofloxacin across bacterial membranes. Like other FQs levofloxacin, FQ-resistant mutants are not easily selected hence resistance develops slowly to FQs. However, increasing resistance has been reported among Salmonella, Pseudomonas, staphylococci, gonococci, pneumococci and C. jejuni.
Due to the unique mechanism of action plasmid mediated transferable resistance perhaps does not occur.
A Qnr protein has been seen that offers protection to the DNA gyrase from damage by the FQs. Also modification of levofloxacin can be caused by a different type acetyltransferase which is similar to the one which modifies aminoglycoside.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence. Although cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Pharmacokinetics
Levofloxacin is absorbed swiftly and to a good extent orally. It attains maximal plasma levels within one to two hours. The bioavailability of levofloxacin is approximately 99-100%. It is extensively distributed into body tissues except in CSF. Plasma protein binding is about 30 to 40%. Only insignificant amounts are metabolised, to inactive metabolites. The elimination half-life of levofloxacin is 6 to 8 hours, which may be prolonged in patients with renal impairment.
These metabolites have little relevant pharmacological activity. Levofloxacin is not removed by peritoneal dialysis or haemodialysis.
Therapeutic uses and dosage
| Condition | Dosage |
| Acute bacterial sinusitis | 500 mg OD for 10 days to one week |
| Acute bacterial exacerbations of chronic bronchitis, Community-acquired pneumonia, Pyelonephritis, urinary system infections, Infections of skin and appendages (complicated) | 500 mg OD for 1-2 weeks |
| Inhalation Anthrax | 500 mg OD for 8 weeks |
| Uncomplicated cystitis | 250 mg OD for three days |
| Chronic prostatitis due to bacteria | 500 mg OD for 4 weeks |
Drug interactions
- Iron, magnesium, zinc or aluminium containing salts
Absorption of levofloxacin is significantly decreased when administered with Fe2+ salts, or Mg2+ or Al+ containing antacids, formulations containing zinc or didanosine.
- Sucralfate
When levofloxacin is administered with sucralfate, its bioavailability is decreased significantly.
- Theophylline, NSAIDs
When levofloxacin is given with theophylline or NSAIDS it lowers the seizure threshold.
- Cimetidine and probenecid
Cimetidine and probenecid inhibit the renal tubular secretion thereby reducing the renal excretion of levofloxacin.
- Vitamin K antagonists
If vitamin K antagonists are prescribed simultaneously with levofloxacin it can lead to increased chances of bleeding.
- QT interval prolongation
Levofloxacin should be cautiously used along with drugs causing QT prolongation.
Special population
Pregnancy
Animal studies have shown damage to the weight bearing joints in the foetus. So, levofloxacin should be avoided in pregnancy.
Breast-feeding
Levofloxacin is secreted during lactation and hence it should be avoided.
Fertility
Animal studies report no impairment in fertility or reproductive system.
Renal impairment
The pharmacokinetics of levofloxacin is affected by renal impairment. Dose should be reduced in patients with renal impairment.
Impaired liver function
As levofloxacin is not metabolised to any significant extent by the liver, dose alterations are not essential in hepatic impairment.
Paediatric population
Levofloxacin is contraindicated in children and growing adolescents
Warnings and precautions
- Tendinopathy and Tendon Rupture
Levofloxacin is linked with an increased risk of inflammation of tendon and rupture of the tendon and it commonly involves the Achilles tendon.
- Hepatotoxicity
Reports of severe liver toxicity have been reported with levofloxacin. However clinical trials have shown no such toxicity.
- Central Nervous System Effects
Convulsions, toxic psychoses are reported in patients receiving levofloxacin. Fluoroquinolones may cause raised intracranial pressure and restlessness and anxious behaviour.
- Clostridium difficile-Associated enterocolitis
Clostridium difficile-associated enterocolitis can be seen with levofloxacin.
- Peripheral Neuropathy
Paresthesias, dysesthesias and weakness have been seen with levofloxacin.
- Prolongation of the QT Interval
Levofloxacin can lead to prolongation of the QT interval and arrhythmia.
- Musculoskeletal Disorders
An increased incidence of joint pain and inflammation and gait abnormality may be seen in children receiving levofloxacin. Levofloxacin is indicated in children (≥6 months of age) only for the prevention of inhalational anthrax (post-exposure).
- Changes in blood glucose level
Increased and decreased blood glucose levels have been seen with levofloxacin, generally in diabetic patients receiving simultaneous antidiabetic medications.
- Photo-toxicity
Moderate to severe photo-toxicity reactions may occur with levofloxacin.
- Patients with G-6- phosphate dehydrogenase deficiency
Patients with glucose-6-phosphate dehydrogenase deficiency are prone to haemolytic reactions.
- Severe bullous reactions
Stevens-Johnson syndrome or toxic epidermal necrolysis have been reported with levofloxacin.
Contraindications
Levofloxacin is contraindicated in following conditions:
- in patients hypersensitive to levofloxacin or other quinolones or any of the excipients
- in patients with epilepsy
- in patients with history of tendon disorders related to fluoroquinolone administration in children or growing adolescents
- during pregnancy
- in breast-feeding women
Adverse effects
The common adverse effects seen with levofloxacin are:
- Giddiness, headache
- Candidiasis
- Sleep disturbances
- Breathlessness
- Vomiting, loose stools, pain in the abdomen
- Skin rashes, increased itching
- Vaginitis
- Oedema, reactions and pain at the injection site
Increased or sometimes decreased blood glucose levels, increased serum potassium levels