 Français
Français Antibiotic Drugs
Levofloxacin
Levofloxacin is a synthetic, broad spectrum, second generation bactericidal fluoroquinolone.
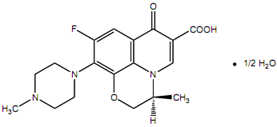
Chemical structure
The empirical formula is C18H20FN3O4 • ½ H2O and the molecular weight is 370.38.
Mechanism of action
Levofloxacin inhibits the enzyme bacterial DNA gyrase and prevents replication of bacterial DNA during bacterial growth and reproduction.
Site of action

Pharmacokinetics
Levofloxacin has nearly complete absorption from gastrointestinal tract. Duration of action is prolonged in patients of renal impairment.
Antimicrobial spectrum
Levofloxacin is effective against wide range of bacteria. Levofloxacin has better action against gram-positive cocci, mycoplasma, legionella, chlamydia than ciprofloxacin.
Indications, administration and dosage
Levofloxacin is effective against sensitive bacteria causing following infections.
| Condition | Adult Dosage/day |
| Nosocomial Pneumonia | 750 mg for 7-14 days |
| Community Acquired Pneumonia | 500-750 mg for 5-14 days |
| Acute Bacterial Sinusitis | 750 mg for 5 days or 500 mg for 10-14 days |
| Acute Bacterial Exacerbation of Chronic Bronchitis | 500 mg for 7 days |
| Complicated Skin and Skin Structure Infections (SSSI) | 750 mg for 7-14 days |
| Uncomplicated SSSI | 500 mg for 7-10 days |
| Chronic Bacterial Prostatitis | 500 mg for 28 days |
| Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP) | 750 mg for 5 days or 250 mg for 10 days |
| Uncomplicated Urinary Tract Infection | 250 mg for 3 days |
| Inhalational Anthrax (Post-Exposure), Adult and pediatric patients > 50 kg and ≥ 6 months of age | 500 mg for 60 days |
| Paediatric patients < 50 kg and ≥ 6 months of age | 8 mg/kg 12 hourly for 60 days |
Precautions, contraindications and warnings
Levofloxacin is contraindicated in patients with known hypersensitivity to levofloxacin or any other fluoroquinolone. Levofloxacin should be cautiously used in pregnancy, nursing women, elderly and in patients with liver or kidney impairment.
Adverse effects
Side effects of levofloxacin are nausea, diarrhea, constipation, abdominal pain, vomiting, dyspepsia, moniliasis, insomnia, headache, dizziness, dyspnea, rash, pruritus, QTc prolongation, tendonitis and tendon repture.Technical Description on Levofloxacin
Levofloxacin is a synthetically derived second generation cephalosporin with action against varied type of bacteria.
Chemical structure
Levofloxacin is a chiral carboxyquinolone that is fluorinated. It is pure (S) enantiomer of ofloxacin which is racemic drug. The molecular formula is C18H20FN3O4 • ½ H2O and MW is 370.38.

Preparations available
Oral: 250, 500, 750 mg tablets; 25 mg/mL solution
Parenteral: 5, 25 mg/mL for IV injection
Ophthalmic : 5 mg/mL solution
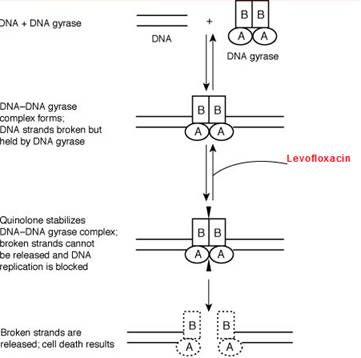
Mechanism of action
Levofloxacin like other fluoroquinolones (FQs) inhibits the enzyme bacterial DNA gyrase that produces a small cut in the double-stranded DNA, leading to formation of negative supercoiling and then re-ligation of the cut ends. This helps in averting positive supercoiling which may occur in excess. The DNA gyrase consists of two A and two B subunits: The A subunit causes small cuts in the DNA, B subunit causes negative supercoiling and then A subunit causes re-ligation. Levofloxacin binds to A subunit with great affinity and restricts the above mentioned functions of the DNA gyrase. In gram-positive bacteria the major target of action is an analogous enzyme topoisomerase IV which nicks and separates daughter DNA strands once the DNA replication is complete. The damaged DNA leads to formation of exonucleases resulting in digestion of the DNA and this possibly contributes to the bactericidal action of levofloxacin.
The mammalian cells possess an enzyme topoisomerase II instead of DNA gyrase or topoisomerase IV that has very low affinity for levofloxacin - thus the low toxicity to host cells.
Microbiology
Levofloxacin is active against:
Aerobic Gram-Positive Microorganisms
- Staphylococcus aureus
- Staphylococcus epidermidis and saprophyticus
- Pneumococci
- Streptococcus pyogenes
Aerobic Gram-Negative Microorganisms
- E. coli
- Haemophilus influenzae and parainfluenzae
- Klebsiella pneumoniae
- Legionella pneumophila
- Proteus mirabilis
- P. aeruginosa
- Moraxella catarrhalis
- Serratia marcescens
- Enterobacter cloacae
Other Microorganisms
- Chlamydophila and Mycoplasma pneumoniae
Resistance
Resistance is mainly because of chromosomal mutation (Quinolone-Resistance Determining Regions {QRDRs}) forming a DNA gyrase or topoisomerase IV with reduced affinity for levofloxacin. Another common mechanism is reduced permeability/increased efflux of levofloxacin across bacterial membranes. Like other FQs levofloxacin, FQ-resistant mutants are not easily selected hence resistance develops slowly to FQs. However, increasing resistance has been reported among Salmonella, Pseudomonas, staphylococci, gonococci, pneumococci and C. jejuni.
Due to the unique mechanism of action plasmid mediated transferable resistance perhaps does not occur.
A Qnr protein has been seen that offers protection to the DNA gyrase from damage by the FQs. Also modification of levofloxacin can be caused by a different type acetyltransferase which is similar to the one which modifies aminoglycoside.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence. Although cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Pharmacokinetics
After oral intake the absorption of levofloxacin is nearly complete and rapid. One to two hours are required to achieve maximal plasma levels. The absolute bioavailability of levofloxacin is approximately 99%, demonstrating complete oral absorption of levofloxacin. It has wide tissue distribution. It is stereo-chemically stable and does not undergoes metabolism to get converted to the original enantiomer i.e. D-ofloxacin. Plasma protein binding is about 30 to 40%. Only insignificant amounts are metabolised. The t1/2 is six to eight hours.
Therapeutic uses and dosage
| Condition | Adult Dosage/day |
| Pneumonia (nosocomial) | 750 mg for a period of 7-14 days |
| Community Acquired Pneumonia | 500 mg for a period of 7-14 days |
| Acute infection of sinuses | 750 mg for five days /500 mg for 10-14 days |
| Acute Exacerbation of Chronic Bronchitis due to bacterial infection | 500 mg for seven days |
| Chronic prostate inflammation due to Bacteria | 500 mg for 28 days |
| Urinary system Infection (complicated) | 250 mg for ten days |
| Urinary system Infection (uncomplicated) | 250 mg for three days |
| Inhalational Anthrax (Post-Exposure), Adult and Children > 50 kg and ≥ 6 months of age | 500 mg for a period of sixty days |
| Children < 50 kg and ≥ 6 months of age | 8 mg per kg 12 hourly for a period of sixty days |
| Infection of skin and appendages (complicated) | 750 mg for 7-14 days |
| Infection of skin and appendages (uncomplicated) | 500 mg for 7-10 days |
All infections mentioned above are caused by the susceptible organism as mentioned in the microbiology section.
Drug interactions
- The oral absorption of levofloxacin is reduced if it is taken within two hours of ingestion of metal cations, antacids or the antiHIV drug didanosine.
- Warfarin
The therapeutic effect of warfarin may be enhanced if used along with levofloxacin.
- Antidiabetic agents
Levofloxacin if prescribed along with antidiabetic agents can lead to decreased blood glucose levels.
Warnings and precautions
- Musculoskeletal Disorders in children
An increased incidence of musculoskeletal disorders i.e. joint pain and inflammation and Tendinopathy has been observed in children receiving levofloxacin. Levofloxacin is indicated in paediatric patients (≥6 months of age) only for the prevention of inhalational anthrax (post-exposure).
- Tendinopathy and Tendon Rupture
Levofloxacin like other FQs can lead to enhanced chances of inflammation of the tendon and also rupture of the tendons.
- Central Nervous System Effects
Levofloxacin like other FQs can lead to seizures, increased intracranial pressure tremors, hallucinations, restlessness, anxious behaviour, confusion, depressive state, vivid dreams, and decreased sleep.
- Clostridium difficile-Associated enterocolitis
It can be seen with levofloxacin like other antibiotics.
- Liver toxicity
Some cases of severe liver toxicity have been seen with levofloxacin during post-marketing surveillance.
- Peripheral Neuropathy
Rarely large or small sized neurons may be affected by polyneuropathy due to levofloxacin leading to altered sensations.
- Prolongation of the QT Interval
Levofloxacin like other FQs can lead to prolongation of the QT interval.
- Blood Glucose Disturbances
Levofloxacin like other FQs can lead to decreased or increased levels of blood glucose particularly in patients with diabetes who are taking an oral antidaiabetic drug or insulin.
- Phototoxicity
Phototoxic reactions can be seen with levofloxacin.
Special populations
- Elderly population
Severe liver toxicity can be seen in persons > 65 years. If elderly patients are using steroids there are enhanced chances of tendinopathy in them.
- Paediatrics
Muscle and skeletal system disorders like joint pain and inflammation with tendonopathy are more commonly seen in children. There are no studies regarding the safety of levofloxacin if it is used for a period of > 14 days. The benefit outweighs the risk only if levofloxacin is used for the post exposure treatment of inhalational anthrax.
- Pregnancy
It is a Category C drug. Levofloxacin was not found to be teratogenic in animal studies.
- Lactation
It is secreted during lactation.
- Kidney dysfunction
Dosage adjustment is needed in patients with impaired kidney function.
- Liver dysfunction
Liver dysfunction does not affect the pharmacokinetics of levofloxacin.
Contraindications
Levofloxacin is contraindicated in persons with a history of hypersensitivity to levofloxacin or any member of the quinolone class of antimicrobial agents.
Adverse reactions
The common adverse effects are:
- Candidiasis
- Sleep disturbances
- Giddiness, headache
- Breathlessness
- Loose stools, sometimes constipation, pain in the abdomen, vomiting
- Skin rashes, increased itching
- Vaginal inflammation
Less commonly seen adverse effects are:
- Decreased hemoglobin, decreased platelet count
- Hypersensitive reactions
Increased or sometimes decreased blood glucose levels, increased blood potassium levels