 Français
Français Antibiotic Drugs
Norfloxacin Eye/Ear Drop
Norfloxacin ophthalmic/ear solution is a synthetic broad-spectrum fluoroquinolone antibacterial available as a sterile isotonic solution.
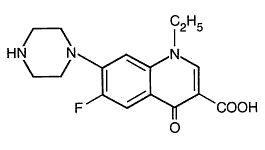
Its empirical formula is C 16 H 18 FN 3 O 3 and MW is319.34.The structural formula is:
Mechanism of action
Ofloxacin inhibits the enzyme bacterial DNA gyrase and prevents replication of bacterial DNA during bacterial growth and reproduction.
Antimicrobial spectrum
Norfloxacin is active against many gram-positive bacteria and gram-negative bacteria. Norfloxacin is not active against obligate anaerobes.
Indications and uses
Norfloxacin ophthalmic solution is indicated for the treatment of acute superficial infections of the eye and its adnexae (conjunctivitis, blepharoconjunctivitis and blepharitis) when caused by the following susceptible bacteria in adults and children:
- Acinetobacter calcoaceticus
- Aeromonas hydrophila
- Haemophilus influenzae
- Proteus mirabilis
- Pseudomonas aeruginosa
- Serratia marcescens
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus warnerii
- Streptococcus pneumoniae
Dosage and administration
| Condition | Adults and paediatric patients (> 1 year) |
| Ophthalmic or eye infection | 1 or 2 drops four times/day for 7 days |
Depending on the severity of the infection, the dosage for the first day of therapy may be one or two drops every two hours during the waking hours.
Precautions, contraindications and warnings
The solution available is not for injection into the eye. Norfloxacin ophthalmic can cause the development of crystals on contact lenses. After applying this medication, wait about 15 minutes before reinserting contact lenses. Do not use any eye drop that is discoloured or has particles in it. Inappropriate use can lead to overgrowth of nonsusceptible organisms including fungi resulting in super-infection.
It is contraindicated in patients with a history of hypersensitivity to norfloxacin, or the other members of the quinolone group of antibacterial agents or any other component of the ophthalmic solution.
Adverse effects
Local burning or discomfort, conjunctival hyperaemia, photophobia, bitter taste following instillation, blurred vision, eyelid itching and swelling.
Technical Description on Norfloxacin Eye/Ear Drop
Norfloxacin ophthalmic/ear solution is a synthetic broad-spectrum fluoroquinolone carboxylic acid antibacterial agent available as a sterile isotonic solution.
Chemical structure
Norfloxacin, a fluoroquinolone, is different from other quinolones as it has a fluorine atom at the 6 position and a piperazine moiety at the 7 position. Its empirical formula is C 16 H 18 FN 3 O 3 and MW is319.34.The structural formula is:

Preparations available
Ophthalmic Solution 0.3% - Each mL contains 3 mg norfloxacin.
One drop contains 0.12 mg of norfloxacin
Mechanism of action
Norfloxacin is bactericidal and it inhibits bacterial deoxyribonucleic acid synthesis. At the molecular level, three particular events are attributed to norfloxacin in E. coli cells:
1) Inhibition of the ATP-dependent DNA supercoiling reaction catalyzed by DNA gyrase,
2) Inhibition of the relaxation of supercoiled DNA,
3) Promotion of double-stranded DNA breakage.
The fluorine atom at the 6 position offers increased potency against gram-negative organisms, and the piperazine moiety at the 7 position is accountable for antipseudomonal activity.
The mammalian cells possess an enzyme topoisomerase II instead of DNA gyrase or topoisomerase IV that has very low affinity for moxifloxacin - thus the low toxicity to host cells.
Microbiology
Norfloxacin ophthalmic solution is effective against the following organisms:
Gram-positive bacteria
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus warnerii
- Streptococcus pneumoniae
Gram-negative bacteria
- Acinetobacter calcoaceticus
- Aeromonas hydrophila
- Haemophilus influenzae
- Proteus mirabilis
- Pseudomonas aeruginosa
- Serratia marcescens
Norfloxacin is not active against obligate anaerobes.
Resistance
Resistance of ophthalmic solution has not been studied, but it is expected to be comparable with oral preparations. Like other fluoroquinolones, resistance is mainly because of chromosomal mutation (Quinolone-Resistance Determining Regions {QRDRs}) forming a DNA gyrase or topoisomerase IV with reduced affinity for norfloxacin. Another common mechanism is reduced permeability/increased efflux of norfloxacin across bacterial membranes. Like other FQs norfloxacin, FQ-resistant mutants are not easily selected hence resistance develops slowly to FQs.
Due to the unique mechanism of action of fluoroquinolones plasmid mediated transferable resistance perhaps does not occur.
Recently two types of plasmid-mediated resistance have been reported.
- The first type utilizes Qnr proteins, which protect DNA gyrase from the fluoroquinolones.
- The second is a variant of an aminoglycoside acetyltransferase capable of modifying norfloxacin.
Both mechanisms confer low-level resistance which may facilitate the point mutations that confer high-level resistance. Resistance to one fluoroquinolone, particularly if it is of high level, usually confers cross-resistance to all other members of the class.
Resistance to norfloxacin due to spontaneous mutation in vitro is rare. Resistant organisms have appeared during therapy with norfloxacin in less than 1% of patients treated.
Usually there is no cross-resistance between norfloxacin and other classes of antibacterial agents. Hence, norfloxacin may show activity against indicated organisms resistant to some other antimicrobial agents including the aminoglycosides, penicillins, cephalosporins, tetracyclines, macrolides, and sulfonamides, including combinations of sulfamethoxazole and trimethoprim. Antagonism has been seen in vitro between norfloxacin and nitrofurantoin.
Therapeutic uses
Norfloxacin ophthalmic solution is indicated for the treatment of acute superficial infections of the eye and its adnexae (conjunctivitis, blepharoconjunctivitis and blepharitis) when caused by the following susceptible bacteria in adults and children:
- Acinetobacter calcoaceticus
- Aeromonas hydrophila
- Haemophilus influenzae
- Proteus mirabilis
- Pseudomonas aeruginosa
- Serratia marcescens
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus warnerii
- Streptococcus pneumoniae
Dosage and administration
| Condition | Adults and paediatric patients (> 1 year) |
| Ophthalmic or eye infection | 1 or 2 drops four times/day for 7 days |
Depending on the severity of the infection, the dosage for the first day of therapy may be one or two drops every two hours during the waking hours.
Drug interactions
Specific drug interaction studies have not been conducted with norfloxacin ophthalmicsolution.
However, the systemic administration of norfloxacin has shown the following interactions which should be kept in mind while using the ophthalmic solution.
- Use of fluoroquinolones including norfloxacin can lead to increase in plasma levels of theophylline when used concurrently.
- Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with norfloxacin.
- Quinolones, including norfloxacin, may enhance the effects of oral anticoagulants, including warfarin or its derivatives or similar agents.
- Quinolones including norfloxacin when given with glyburide can rarely lead to severe hypoglycaemia.
- Like other fluoroquinolones norfloxacin inhibits CYP1A2 in vitro. When used with other drugs metabolized by CYP1A2 like caffeine, clozapine, ropinirole, tacrine, theophylline, tizanidine it may lead to increased substrate drug concentrations when given in normal doses.
- Norfloxacin can interfere with the metabolism of caffeine which may result in decreased clearance of caffeine and a prolongation of the plasma half-life.
- Non-steroidal anti-inflammatory drug (NSAID) and norfloxacin given together may enhance the risk of CNS stimulation and convulsive seizures.
Warning and precautions
The solution available is not for injection into the eye.
- Norfloxacin ophthalmic can cause the development of crystals on contact lenses. After applying this medication, wait about 15 minutes before reinserting contact lenses.
- Do not use norfloxacin ophthalmic if there is a viral or fungal infection of the eye.It is used to treat infectionscaused by bacteria only.
- Do not use any eye drop that is discoloured or has particles in it.
There are no other specific warnings for the ophthalmic solution, but the precautions for the oral solution should be kept in mind.
- Hypersensitivity Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been seen in patients receiving quinolone therapy, including norfloxacin.
- As with other antibacterial preparations, use for a long time can lead to overgrowth of nonsusceptible organisms including fungi resulting in super-infection.
- According to clinical condition, the patient should be examined with the aid of magnification, such as slit lamp biomicroscopy and, where suitable, fluorescein staining.
- Bacterialkeratitishas been seen associated with the use of multiple dose containers of topical ophthalmic products. Usually these containers had been inadvertently contaminated by patients who, in most cases, had a concomitant cornealdisease or a disruption of the ocular epithelial surface.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not shown any concrete proof of carcinogenesis, mutation or impairment of fertility by Moxifloxacin.
Special population
- Pregnancy
It is a pregnancy Category C drug. Oral norfloxacin has not shown any teratogenic effect in most of the animal studies. There are no adequate and well-controlled studies in pregnant women. Norfloxacin Ophthalmic Solution should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
- Nursing Mothers
It is not known whether norfloxacin is excreted in human milk following ocular administration. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from norfloxacin, appropriate decision should be taken.
- Paediatric Use
Safety and effectiveness in infants below the age of one year have not been established.
Although quinolones including norfloxacin have been shown to cause arthropathy in immature animals after oral administration, topical ocular administration of other quinolones to immature animals has not shown any arthropathy and there is no evidence that the ophthalmic dosage form of those quinolones has any effects on the weight-bearing joints.
- Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and young patients.
Contraindication
It is contraindicated in patients with a history of hypersensitivity to norfloxacin, or the other members of the quinolone group of antibacterial agents or any other component of the ophthalmic solution.
Adverse effects
The most commonly observed adverse effects were:
- Local burning or discomfort
- Conjunctival hyperaemia
- Chemosis
- Photophobia
- Bitter taste following instillation
- Blurred vision
- Eyelid itching and swelling